Temporal relationship between atherogenic dyslipidemia and ... - BMC Medicine
Study participants
Initiating in 2006, the Kailuan Study (trial registration number: ChiCTR–TNC–11001489) was an ongoing, prospective, cohort study conducted in Tangshan, China. The following health surveys were issued every 2 years, with a total of seven surveys through to December 31, 2020. Details of the study design have been provided previously [19, 20]. Each participant provided written informed consent before enrollment. The current subanalysis of the Kailuan Study was approved by the Kailuan General Hospital Ethics Committee, China (2006–05) and the Human Research Ethics Committee of Edith Cowan University (2021–03159–BALMER).
Fig. 1 displays the flowchart of participant enrollment in this current study. Among 101,510 participants who attended the first survey in 2006/2007, we excluded a total of 49,285 participants, including those who did not attend the following two health surveys (n = 43,583); those who had incomplete information on sex, age, or abnormal lipid profiles and high-sensitivity C-reactive protein (hsCRP) data (n = 3031); and those who missed any of the follow-up visits (n = 2671), leaving 52,225 participants in the path analysis. We further excluded those with preexisting diabetes before the commencement of follow-up (2010/2011) (n = 8865) for the survival analysis. Finally, a total of 43,360 participants were included in the prospective analysis of the T2D outcome. The number of participants and participations in the four follow-up visits and participants who attended each follow-up visit are reported in Additional file 1: Table S1.
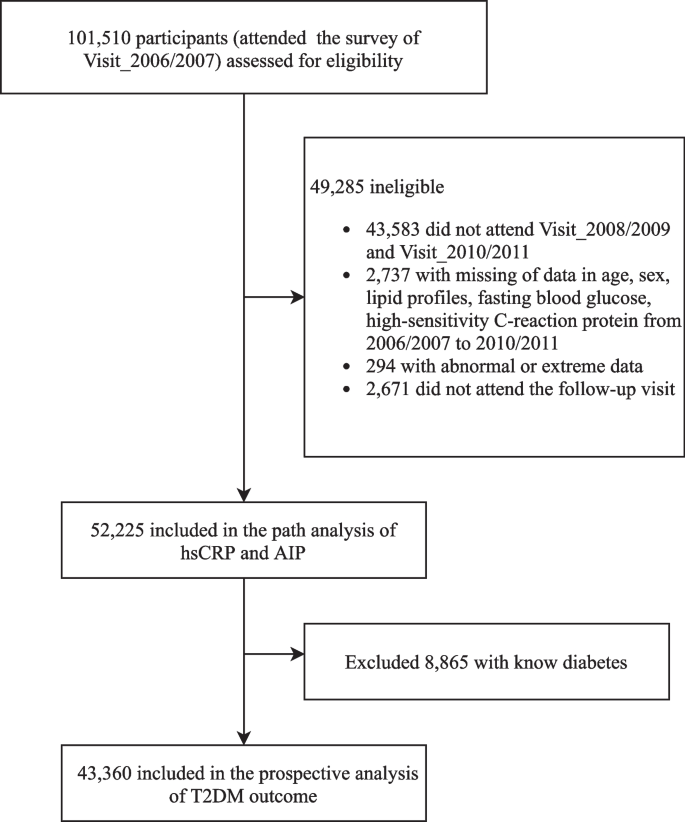
Flowchart of the study participants
Ascertainment of outcome
The primary outcome of this study was the incidence of T2D (International Classification of Diseases–10 [ICD–10]: E11). T2D was diagnosed as either fasting blood glucose (FBG) ≥ 7.0 mmol/L, a self-reported history of T2D diagnosis, or self-reported medication uses of oral antihyperglycemic agents [21]. The date of T2D onset was defined as the first of the available follow-up examinations at which a participant met the diagnostic criteria. Participants contributed their follow-up time until the occurrence of T2D or death or the last available follow-up visit.
Exposure assessment
A brief summary of the study design and procedures of this cohort is provided in Fig. 2. The cumulative exposure was attained in a median 3.95 [interquartile range (IQR): 3.73–4.29] years of period predating the follow-up. Chronic inflammation was measured by cumulative hsCRP (CumCRP), calculated as (hsCRP_2006/2007 + hsCRP_2008/2009)/2 × (visit 1 − 2) + (hsCRP_2008/2009 + hsCRP_2010/2011)/2 × (visit 2 − 3), where hsCRP_2006/2007, hsCRP_2008/2009, and hsCRP_2009/2010 were the hsCRP levels measured at 2006/2007, 2008/2009, and 2010/2011 physical examinations, respectively, and visit 1 − 2 and visit 2 − 3 indicated the time intervals between the two health surveys. Cumulative AIP (CumAIP) and other lipid characteristics were calculated using the same algorithm. As no clear threshold for cumulative hsCRP currently exists and the high intraindividual variability over time supports repeat measurements for ensuring a stable assessment, we used the suggested clinical cutoffs (< 1, 1 to 3, ≥ 3 mg/L) for transient hsCRP in the Asian population [22, 23] as the CumCRP thresholds. The comparison of CumCRP and CumAIP to the mean value of each transient measure in the exposure period is displayed in Additional file 1: Table S2.
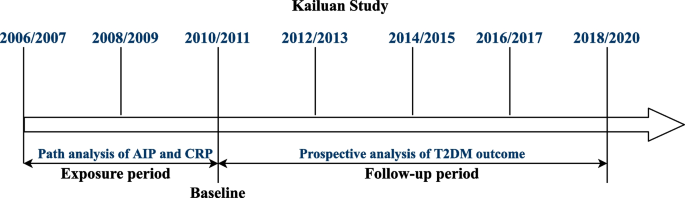
Strategies and design of the current study. The health examinations in the Kailuan Study were provided around every 2 years, except for the current last visit, with a time span of approximately 3 years owing to the influence of the COVID-19 pandemic. For the current study, the path analysis addressing the temporal relationship between AIP and hsCRP was based on data measured in 2006/2007 and 2010/2011. For the survival analysis of T2DM outcome, the cumulative exposure period was from 2006/2007 to 2010/2011. At the end of Visit_2010/2011, the nondiabetic participants were followed up biannually through December 31, 2020. Baseline characteristics were based on the information in Visit_2010/2011
Covariates
As described previously [20], the data on sociodemographic characteristics, anthropometric measurements, biochemistry parameters for evaluating systemic health (lipid profiles, hsCRP, creatine, FBG), and lifestyle factors (alcohol consumption, smoking) as well as past medical and medication history (diabetes, CVD, hypertension, fatty liver, and current treatments including antihypertensives, antidiabetic, and lipid-lowering agents) were collected via standardized questionnaires. Anthropometric measurements of height, weight, and blood pressure were conducted by trained physicians following a standard protocol. Blood pressure was categorized as normal blood pressure, grade I hypertension, grade II hypertension, and grade III hypertension [24]. Fatty liver was routinely assessed by abdominal ultrasonography by experienced radiologists using a high-resolution B-mode topographical ultrasound system with a 3.5-MHz probe (ACUSON X300, Siemens, Germany). The severity of fatty liver was categorized as mild, moderate, or severe. The estimated glomerular filtration rate (eGFR) was calculated from creatinine levels following the Chronic Kidney Disease Epidemiology Collaboration formula [25]. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Current smokers were defined as smoking at least one cigarette/day on average in a recent year. Drinking status was defined according to average alcohol consumption in the past year.
Statistical analysis
Multiple imputation by chained equation techniques was performed for missing values in potential covariables (> 98% complete). We examined the temporal relationship between hsCRP and AIP in the exposure period (median period of 3.95 years) with a typical cross-lagged panel design, as demonstrated in Additional file 1: Fig. S1. This design measured the path coefficient (β1) of hsCRP_2006/2007 on AIP_2010/2011 and the path coefficient (β2) of AIP_2006/2007 on hsCRP_2010/2011 simultaneously, adjusting for the auto-regressive effects. A significant path coefficient (β1 or β2) suggests directionality, and a significant difference between β1 and β2 provides stronger evidence for the directional path between the two variables measured over time. Statistical differences in β1 and β2 were examined using a t test. In this analysis, hsCRP was log-transformed, and then both log (hsCRP) and AIP were standardized to means as 0 and standard deviation (SD) as 1. The multivariable-adjusted models were as follows: model 1 was adjusted for age, sex, lipid-lowering drugs, antihypertensive drug use, smoking habits, alcohol consumption, BMI, FBG, systolic blood pressure (SBP), eGFR, and LDL-C measured in 2006/2007, and model 2 was further adjusted for the time interval between examinations (years).
For baseline descriptions of the nondiabetic participants in the prospective analysis of T2D risks, the mean and SD, median and IQR, or frequency and percentage (%) were used, as appropriate. Differences in the baseline characteristics across the six CumAIP-by-CumCRP strata were compared by ANOVA (for continuous normally distributed variables), Kruskal–Wallis test (for continuous skew-distributed variables) and χ2 test (for categorical variables). Because of skewed distribution, CumCRP, hsCRP, HDL-C, and TG were log-transformed as continuous variables, and eGFR was divided into 4 categories according to clinical cut-points: ≥ 90, 60~90, 30~60, and < 30, in the model analyses.
Incidence rates of T2D were calculated as per 1000 person-years. The association between CumAIP and T2D incidence, with or without stratifying by CumCRP thresholds, was examined utilizing multivariable Cox proportional hazards regression models. The association between CumCRP alone and incident T2D was investigated using weighted Cox proportional hazards models because of the violation of the proportional hazards' assumption. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. P values for trend and risks per SD increment of CumAIP or log (CumCRP) were calculated. The multiplicative interaction (INTm) between CumAIP and CumCRP was tested with the likelihood ratio test. The joint effect of CumAIP and CumCRP on the risk of developing T2D was further investigated using multivariable-adjusted models: model 1 was adjusted for age, sex, education, smoking, alcohol consumption, physical activities, family history of diabetes, and BMI; model 2 was further adjusted for FBG, eGFR, total cholesterol (TC), blood pressure, antihypertensives (yes or no), and lipid-lowering drugs (yes or no); and model 3 was additionally adjusted for fatty liver degree. To compare the overall survival of each risk group of CumAIP-by-CumCRP strata based on follow-up intervals, the Kaplan–Meier plots were generated, and the log-rank test was conducted. Further INTm analyses were performed between joint cumulative exposure and baseline sex, overweight, hypertensive, dyslipidemia, or impaired fasting glucose (IFG) status. Stratified analyses among these covariates were performed according to the identified interactions. In addition, sensitivity analyses were performed to assess the robustness and consistency of the findings. Firstly, we excluded participants with baseline CVD to minimize the influence of potential confounds on the lipid and inflammation biomarkers and T2D risk. Secondly, we excluded study endpoints that occurred within the first follow-up visit to address the potential reverse causation. Thirdly, we excluded those with any hsCRP level ≥ 10 mg/L during the exposure period. Fourthly, we performed an analysis with the raw datasets (without imputation for missing values). Fifthly, since the data with the study endpoint of T2D were interval-censored survival datasets, we additionally performed the analyses with the SAS ICPHREG procedure to fit a proportional hazards model on the interval-censored survival data. Sixthly, we further performed the survival analyses by adjusting for the time-varying covariates in the follow-up period. At each date of an event (T2D), the model used the covariates present at the visit just before the event.
All statistical analyses were conducted in the SAS software (version 9.4; SAS Institute, Cary, NC) with the SAS Proc Calis procedure for cross-lagged analysis. A two-sided P value < 0.05 was considered statistically significant, except for a P value < 0.1 in the interaction testing.
Comments
Post a Comment